Test Data
LABORATORY
Antimicrobial Performance
A UKAS approved laboratory study to determine the efficacy of medixair against three strains of bacteria. Download here.
This study has focused on determining the microbiological performance of the Medixair device by investigation of the device in the three following areas;
a. In vitro verification of the antimicrobial potential of the energy generated by a single UVc lamp employing surrogate cultures for Bacillus anthracis ( as spores) and other class II pathogens in vegetative form
b. In vitro volumetric antimicrobial performance in relation to the removal of surrogates for Bacillus anthracis (as spores).
c. Performance in an environmental situation-employing surrogate cultures for Bacillus anthracis (as spores) and other class II pathogens.

“[the] data illustrates that the Medixair device represents a significant advance in atmospheric treatment. Our data suggests a sensible and effective combination of airflow rate to UVc dosage has been conceived in a system, which should integrate efficiently, and effectively into environmental biohazard protection systems” – Microsearch UK Ltd.
Medixair MRSA Test
Antimicrobial Performance of the Medixair UVc Air Sterilisation Device in the Sterilisation of Three Strains of Staphylococcus. Download here.
a study of the medixair UVc air sterilisation unit. In relation to three strains of Staphylococcus aureus which are off considerable medical significance. The Medicare 15 unit is a multi lamp UVc emitter which passes air through a decontamination chamber by means of a fan and which is intended for use in atmospheric control of Biohazards.
The following organisms were employed in the trial;
- Staphylococcus aureus; NCTC 11939; Carries gentamicin and Chloramphenicol plasmids/Epidemic methicillin resistant strain
- Staphylococcus aureus; NCTC 11940 ; Epidemic methicillin resistant strain
- Staphylococcus aureus; NCTC 11962 ; Associated with post operative toxic shock

“We have further demonstrated that the Medixair device is capable of achieving between 6.6 and 7.2 log cycles of kill over an eight-hour period. Again this was demonstrated by employing very high numbers of organisms in atmospheric dispersion and such a performance therefore represents a very positive indicator that the device presents as a valuable utility in any strategy designed to control Staphylococcal contamination of medical environments.” – Microsearch UK Ltd
Antiviral Report
Evaluation of the anti-viral performance of the Medixair UVc device. Download here.
In this study we have we have investigated the performance of the Medixair UVc air sterilisation unit in the inactivation of four common virus groups which cover particles composed of either
double strand DNA, single strand DNA, double strand RNA or +single strand RNA.

“Medixair was effective in reducing continuous doses of each of four viruses over a 4.5-hour period. The data illustrates a sustained level of inactivation between, 99.996% and 99.9995%” – Microsearch UK Ltd
Antibacterial Test in a Low Ventilation Environment
Removal of airborne Serratia marcescens in a controlled indoor environment using medixair UV device. University of Leeds aerosol test chamber. Download here.
A series of trials were undertaken in order to determine the efficacy of a boxed UV device in the removal of airborne microorganisms (Serratia marcescens) from a controlled indoor environment.
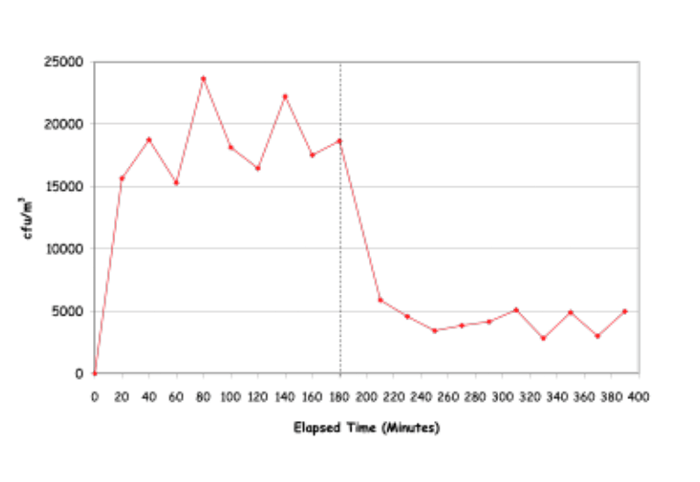
79% Removal of Serratia marcescens at 1.5 AC/hour using two medixair units mounted on the long wall of the room.
The trial was carried out over 6.5 hours during which the microorganisms were nebulised into the room at a constant rate. During the first 3 hours no UV device was After 3 hours the UV device was switched on. Samples were then taken every 20 minutes for another 3 hours to determine the new steady state concentration. The performance of the device in terms of the percentage removal of the microorganism was calculated as the difference between the average concentration before and after.
“Under low ventilation rates the performance increases dramatically to give very good removals.” – Dr. Louise Fletcher
CLINICAL
The Efficacy of a Mobile Air Sterilisation Device (Medixair) on the Airborne Spread of Methicillin-Resistant Staphylococcus aureus
Department of Clinical Microbiology, Northwick Park Hospital, North West London Hospitals NHS Trust
The study measured the effect on MRSA colonisation of patients and surface contamination in a side ward. Download here.
A controlled, sequential clinical trial assessed the efficacy of a new mobile air sterilisation device (Medixair) using ultraviolet light for sterilising the air in a single bed room of a busy general medical ward.
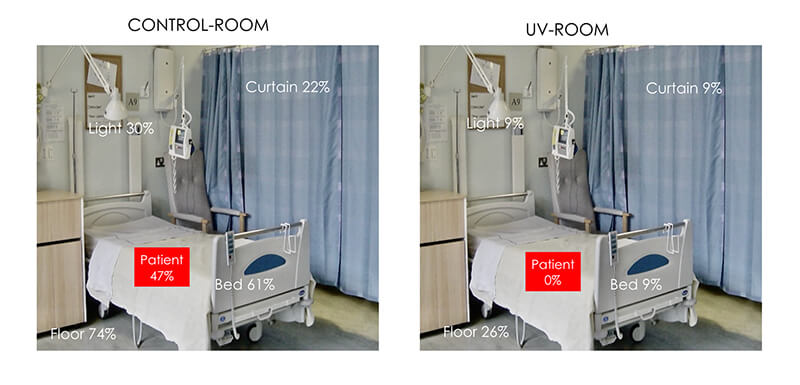
“No Patient Colonisations”
“The study is the first sequential clinical trial testing the effect of ultraviolet light against MRSA in a clinical setting. The study demonstrated Medixair ‘s effectiveness in reducing MRSA contamination from the environment and also its ability to protect patients – even when MRSA was present in the immediate vicinity.” – Dr Peder Bo Nielsen, Consultant Microbiologist
Clinical trial – air sampling in a UK 4 bed high dependency unit of a private hospital
D.O’Connor B.Sc. Ci.Biol M.I.F.S.T. Microsearch UK Ltd
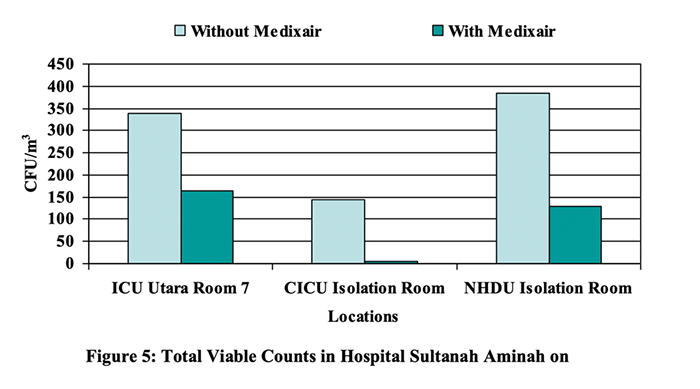
Air sampling before and after the application of medixair in a high dependency ward. Download here.
Two Medixair units used in conjunction with a Cassala air sampler. Period A – a control and Period B a live test . Comparison with an external air measurement and normal ward activity.

A 59% reduction in the bio-burden even when challenged from a higher external input load.
Conclusion
Our data is encouraging in that, over the two periods, it was demonstrated that there was a significant (59%) reduction in the bio-burden even when challenged from a higher external input load.
Included within the observed levels of organisms during Period A was the presence of Staphylococcus aureus. During the second period, Period B with the machine switched on no Staphylococcus aureus was detected.
Queen Elizabeth Hospital Kota Kinabalu Malaysia CCU Wards 1 and 3
Elsa Ng BSc (Hons) Microbiology Initial Healthcare
This report describes the air sampling programme before and after the the deployment of medixair in the Coronary Care Unit Kota Kinabalu, Malaysia. Download here.
Air samples were taken pre and post Medixair installation, using a BIO-TEST RCS Hi Flow Air Sampler and testing for Total Viable Count TVC of bacterial organisms.
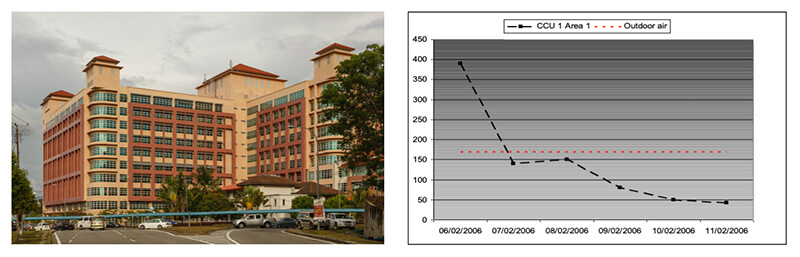
“The data provides a clear indication of the levels of contamination that one would expect to encounter within the subject CCU Wards and the positive effect provided by Medixair in all locations tested.
Of particular note is the fact that the all post Medixair installation results – with the exception of only one sample – almost certainly relating to a single challenge event are below the contamination level for fresh outdoor air.” – Elsa Ng, BSc (Hons) Microbiology
Microbial Air Sampling Report in Three General Hospitals in Malaysia
Elsa Ng BSc (Hons) Microbiology Initial Healthcare
To evaluate and assess the air quality in particular to specific microbial activity before and after installation of Medixair at the selected locations. Download here.
This report describes the microbial air sampling carried out at the above premises. The objective of this study was to evaluate and assess the air quality in particular to specific microbial activity before and after installation of Medixair at the selected locations.
“The results showed that the initial total viable counts in the rooms in the Burn Care Ward were between 35 and 245 CFU/m3. Relatively high total viable counts that ranged between 735 and 910 CFU/m3 were recorded in the cubicles sampled in CCU on 03 September 2007. After Medixair was installed, marked reductions in total viable counts that ranged from 14.3% to 89.0% except for Burn Care Ward Room 2, which recorded an increase.” – Elsa Ng, BSc (Hons) Microbiology
Aerobiology and nosocomial transmission of Clostridium difficile
Department of Clinical Microbiology, Northwick Park Hospital, North West London Hospitals NHS Trust
As an interventional measure it was decided to place some newly acquired ultraviolet air sterilisation units in an acute trauma ward. Download here.
As part of a hospital “clean air programme” air sterilisation units were introduced. It had previously proved efficient against MRSA in controlling the spread and protecting patients.
The acute trauma ward suffered a severe outbreak of diarrhoea and vomiting hence it was decided to equip it with ten Ultraviolet air sterilisation units (Medixair).
The first quarter reduced the number of CDI cases from 12 to 4, and the subsequent three quarters had either none or just one case of CDI.
The hospital as a whole demonstrated a modest reduction of the total number of CDI cases, but not to the same degree as in the acute orthopaedic trauma ward.
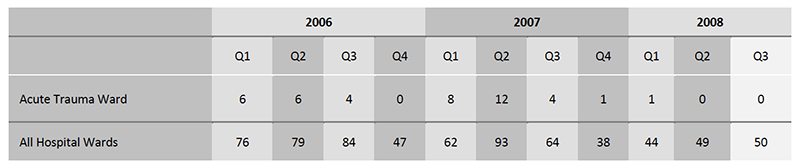
It is seen that the hospital reduced the average number of CDI cases per six months from 146, a reduction of 33% On the same basis the trauma ward achieved a 80% reduction from 12 cases per six months to 2.4 cases.
In conclusion, ultraviolet air sterilisation seems to reduce the number of CDI outbreaks and the number of cases.
It is hypothesised that the airborne mode of transmission plays a role in transmission of Clostridium difficile, just as it has been demonstrated for MRSA.
Dental – Measurement of Airborne Micro-organisms in Five French Practices
Pathogen Solutions and Eurotec Dental, Paris
The air in each of the practices was sampled for airborne microorganisms and the effect of Medixair. Download here.
The air in each of the practices was sampled for airborne microorganisms. In each establishment a series of agar strips were exposed using a Biotest HCS air sampler. Agars for the identification of coliforms and staphylococcus were employed together with a general agar for determination of total viable count.
Medixair units were then installed in each surgery and allowed to run overnight. The air was then re-sampled the next day. The results are given below. A significant reduction in airborne microorganisms was demonstrated.

“These results are consistent with those obtained by Pathogen Solutions Limited in doctor’s surgeries, nurseries, hospital wards and laundries. They demonstrate clearly the effectiveness of Medixair in eliminating airborne microorganisms. Extending the period between the pre and post test would have given even further reductions.” – Geoffrey Smith, Business Development Director, Pathogen Solutions
